1 Stain the Maize leaves with developed tumor symptome due to the inoculation of
Ustilago
Cut the Maize leaves showing disease symptom into several piecies, distained in the Ethoh: Acetic Acid=2:1 solution overnight. Then poured out the distaining solution and stained the leaves in the Trypan Blue Dye in lactophenol for 6 hr, then distained the leaves for 1 hr. Check the sample under the compound microscope.
Trypan Blue staining Maize leaves
The Trypan Blue can stain both dead cells and fungal structures. Under the microscope we can see there are indeed existence of
Ustilago in the tumor of the Maize leaves.
2 Observe all kinds of spores in Ascomycota
2.1
Tricoderma viride (Ascomycota/Sordariomycetes/Hypocreomycetidae)
Trichoderma viride is a fungus and a biofungicide. It is used for seed and soil treatment for suppression of various diseases caused by fungal pathogens. It is also a pathogen causing green mould rot of onion.
Pic 1. Tricoderma viride Chlamydospore
The bigger spore I saw should be Chlamydospore, which is a kind of resting cell, more resistant to adverse environment.
Pic 2. Tricoderma viride conidia
The smaller spore I saw should be conidia, small, round.
2.2
Pestalotia Sp. (Ascomycota/Sordariomycetes/Xylariomycetidae)
Pestalotia is primarily a secondary pathogen. It is saprophytic on dead and dying tissues and is weakly parasitic infecting wounds under moist conditions, causing important diseases- Pestalotiopsis tip blight of conifers.
Pic 3. disease syptome of tip blight of conifers (from website)
Pic 4. Petalotia. sp. conidia
Conidia are multi-celled with usually three darkly pigmented center cells and clear pointed end cells. Conidia are ellipsoid or fusoid (football-shaped). A diagnostic feature is the two or more clear, whisker-like appendages arising from the end cell.
http://plantpath.caes.uga.edu/extension/Fungi/pestalotia.html
2.3
Fusarium graminearum (Dikarya/Ascomycota/Pezizomycotina/Sordariomycetes/Hypocreales)
The name of
Fusarium comes from Latin
fusus, meaning a spindle.
Fusarium is a large genus of filamentous fungi widely distributed in soil and in association with plants. Most species are harmless saprobes, and are relatively abundant members of the soil microbial community.
Some species produce mycotoxins in cereal crops that can affect human and animal health if they enter the food chain. The main toxins produced by these Fusarium species are fumonisins and trichothecenes.
F. graminearum can also cause root rot and seedling blight. The total losses in the US of barley and wheat crops between 1991 and 1996 have been estimated at $3 billion.
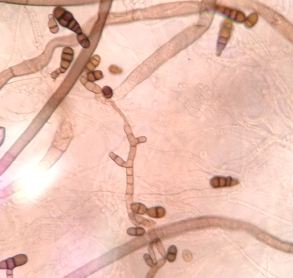
Pic 5. Fusarium graminearum macroconidia
2.4
Colletotrichum coccodes (Ascomycota/Pezizomycotina/Sordariomycetes/Glomerellales)
Colletotrichum coccodes is a plant pathogen, which causes anthracnose on tomato and black dot disease of potato. Many of the species in this genus are plant pathogens, although some species may express a mutualistic life-style in non-disease hosts as obligate symbionts to plants in the form of endophytes.
Pic 6. Colletotrichum coccodes conidia
Colletotrichum coccodes conidia are straight, fusiform, attenuated at the ends.
Pic 7. Colletotrichum coccodes sclerotia with setae
Sclerotia are usually abundant, setose, spherical and are often confluent.
http://www.mycology.adelaide.edu.au/Fungal_Descriptions/Coelomycetes/Colletotrichum/
5.
Alternaria brassicicola (Ascomycota/Dothideomycetes/Pleosporales)
Pic 8. Alternaria brassicicola
Alternaria brassicicola is considered a necrotrophic plant pathogenic fungus and like other
Alternaria species has been shown to secrete numerous toxic secondary metabolites and proteins that cause cell death via induction of apoptosis in plants or by directly damaging cells.
A. brassicicola causes black spot disease on virtually every important cultivated Brassica species including broccoli, cabbage, canola, and mustard.
Alternaria brassicicola has routinely been used as a model necrotrophic fungal pathogen in studies with Arabidopsis thaliana, also a weedy member of the Brassicaceae plant family.
From a human health perspective,
Alternaria brassicicola is representative of a genus of fungi that is considered one of the most potent sources of mold-derived allergens. Moreover, sensitization to
Alternaria is also strongly associated with chronic respiratory diseases such as asthma and chronic rhinosinusitis.
http://genomeportal.jgi-psf.org/Altbr1/Altbr1.home.html
6.
Botrytis Cinera (Ascomycota/Pezizomycotina/Leoitomycetes/Helotiales)
Botrytis cinerea is a necrotrophic fungus that affects many plant species, although its most notable hosts may be wine grapes.
Botrytis cinerea is characterized by abundant hyaline conida (asexual spores) borne on grey, branching tree-like conidiophores. The fungus also produces highly resistant sclerotia as survival structures in older cultures. It overwinters as sclerotia or intact mycelia, both of which germinate in spring to produce conidiophores. The conidia are dispersed by wind and rain-water and cause new infections.
Pic 9. Botrytis cinera